Enables patient-specific immunotherapy by developing precision medical technology centered around organoid-based phenotyping to select the most suitable immune checkpoint inhibitors for each patientValidation of drug response similarity between tumor organoids and patients through clinical trials (started in January 2023)
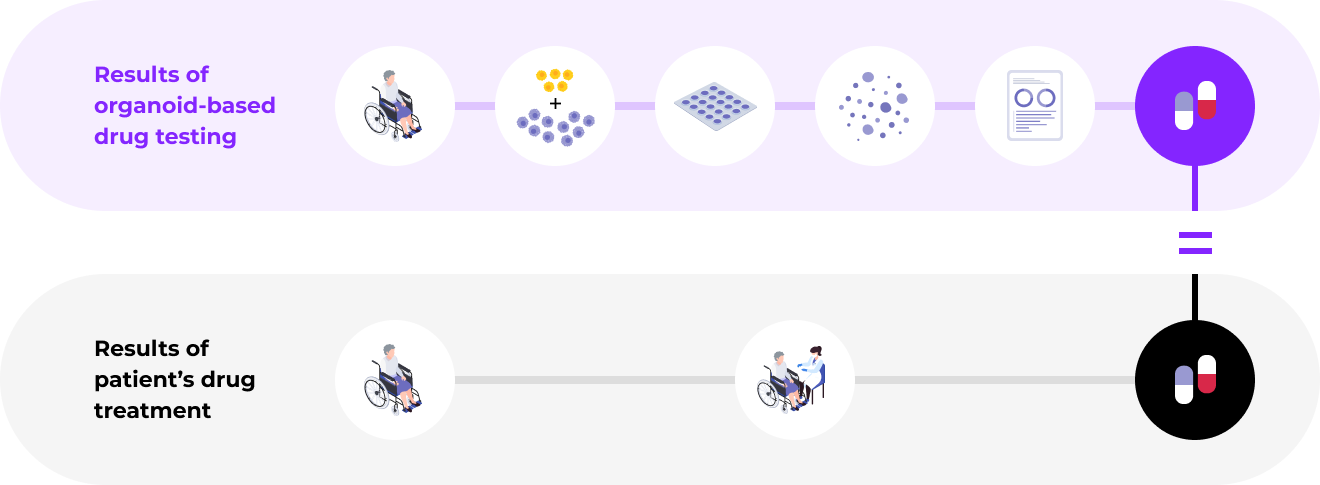
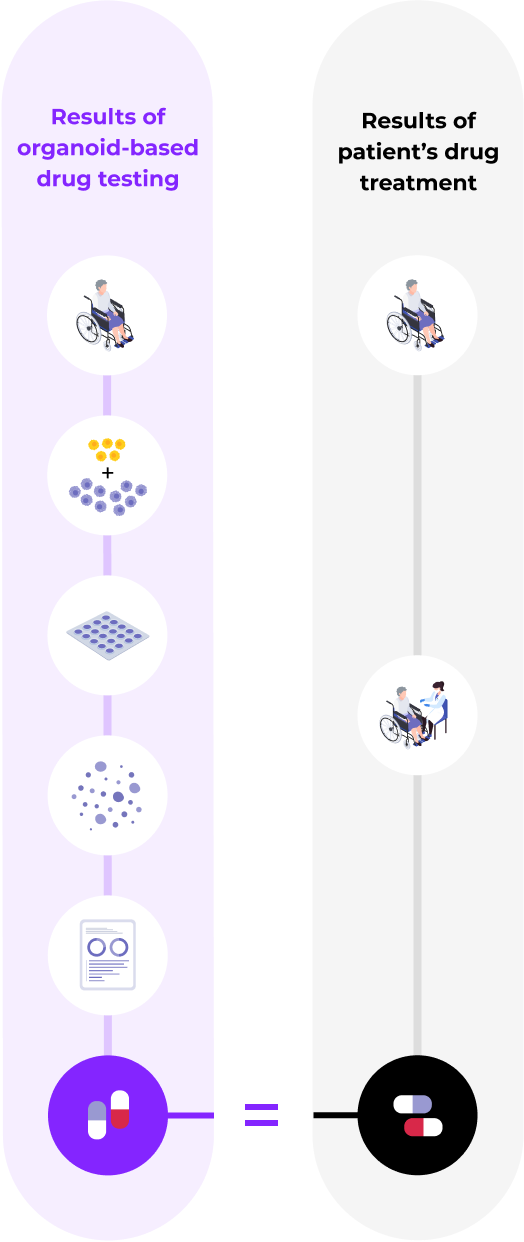
We demonstrated the superiority of the organoid platform
through comparison with actual clinical trials
High predictive level of the organoid-based drug response prediction platform
| Results of test | Disease (O) | Disease (X) | Total |
|---|---|---|---|
| Positive | a | b | a+b |
| Negative | c | d | c+d |
| Total | a+c | b+d | a+b+c+d |
- Sensitivity = [a/(a+c)]x100
- Specificity = [d/(b+d)]x100
- Positive predictive value = [a/a+b]x100
- Negative predictive value = [d/c+d]x100
| Results of test | Disease (O) | Disease (X) | Total |
|---|---|---|---|
| Positive | 7 | 0 | 7 |
| Negative | 0 | 5 | 5 |
| Total | 7 | 5 | 12 |
- Sensitivity = 100
- Specificity = 100
- Positive predictive value = 100
- Negative predictive value = 100
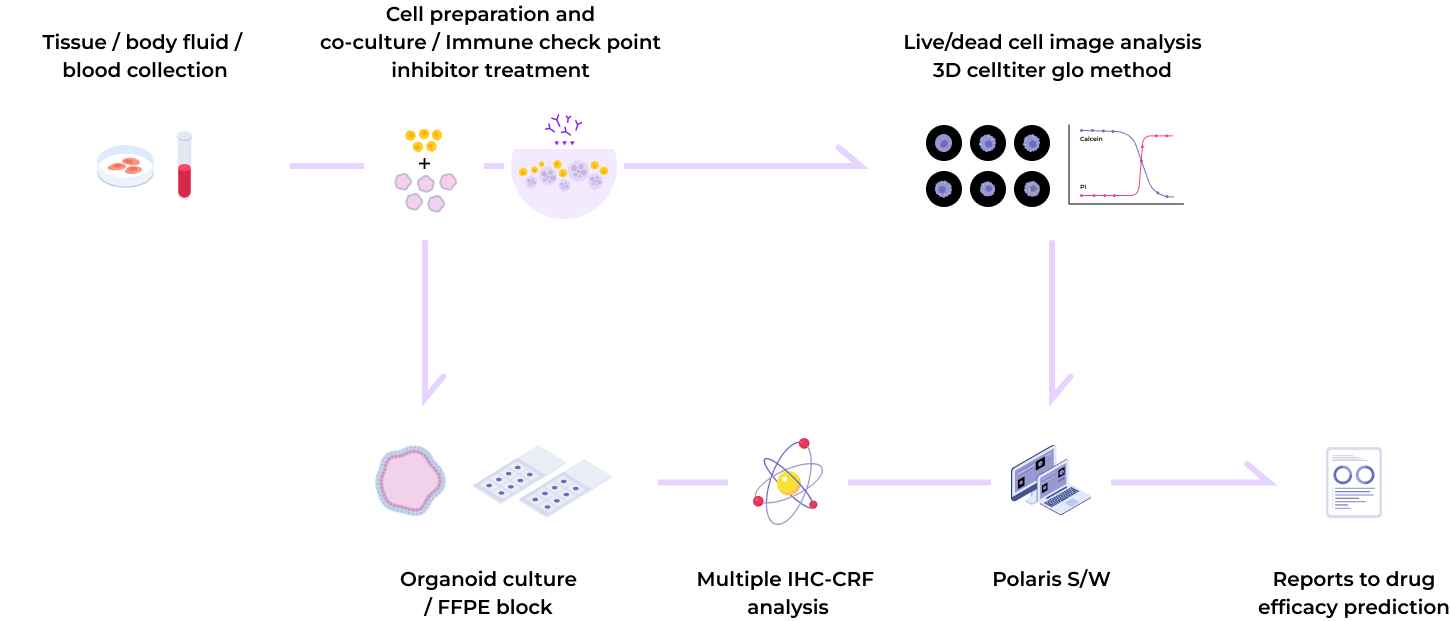
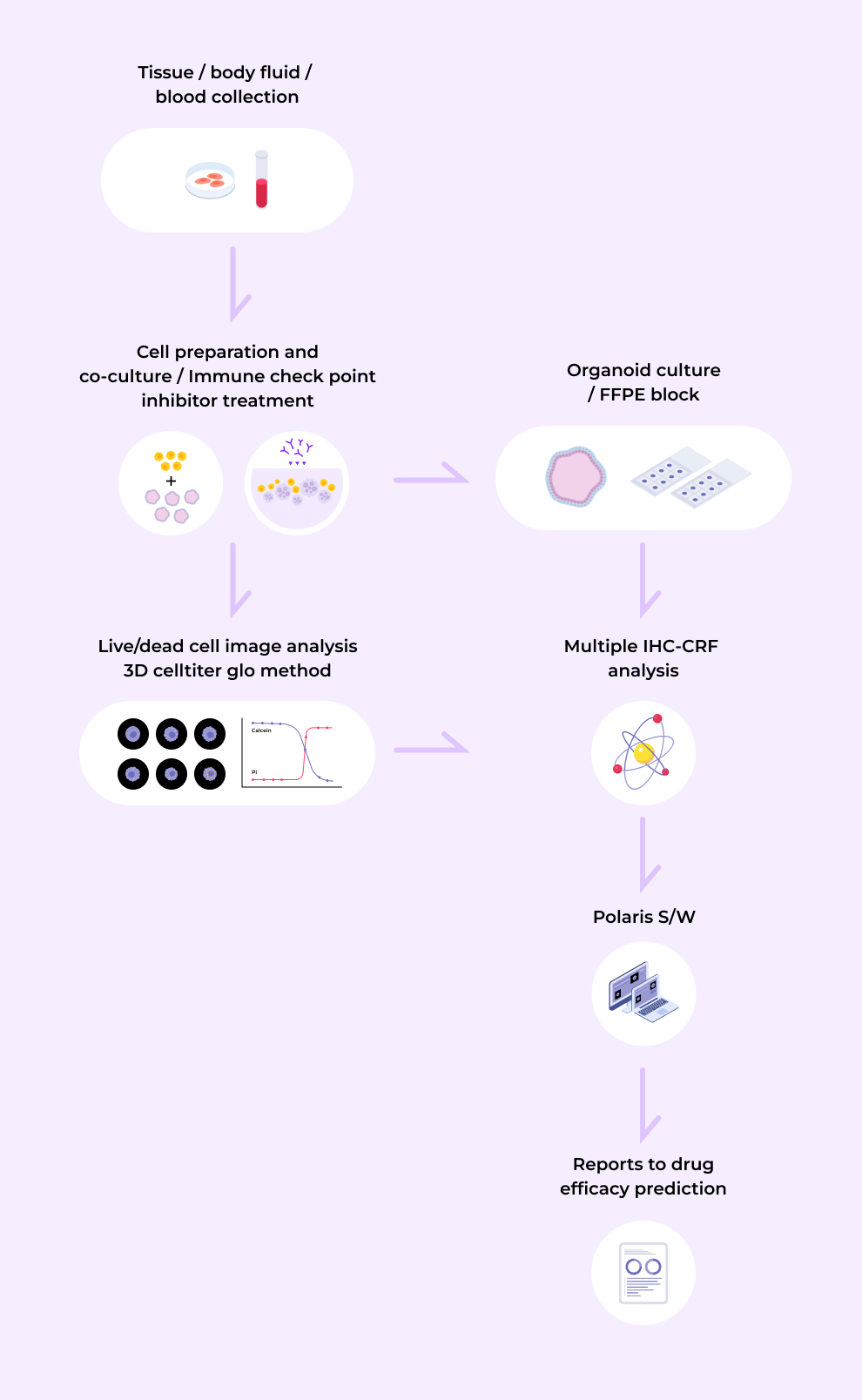
Organoid research incorporating spatial biology technology
By combining organoid technology with spatial biology analysis, our "organoid-based phenotypic companion diagnostics technology" enables the selection and evaluation of multiple biomarkers and drugs
-
Step 01
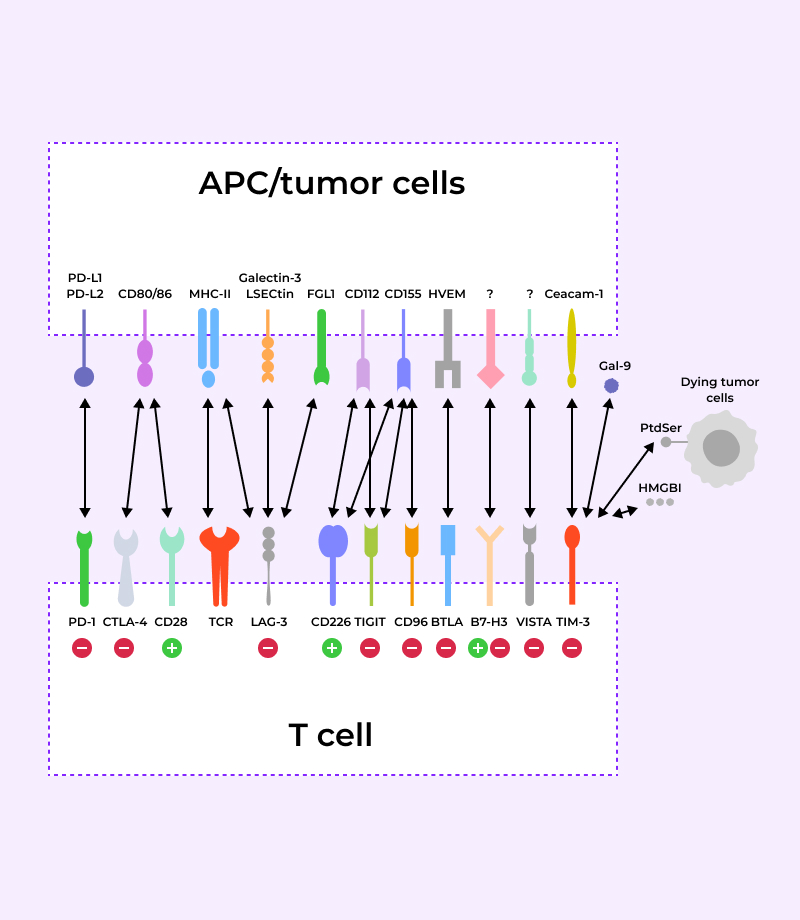
Immune checkpoint factor staining with Multiplex-IHC
-
Step 02
Confirmation of immune checkpoint factor expression and target selection
-
Step 03
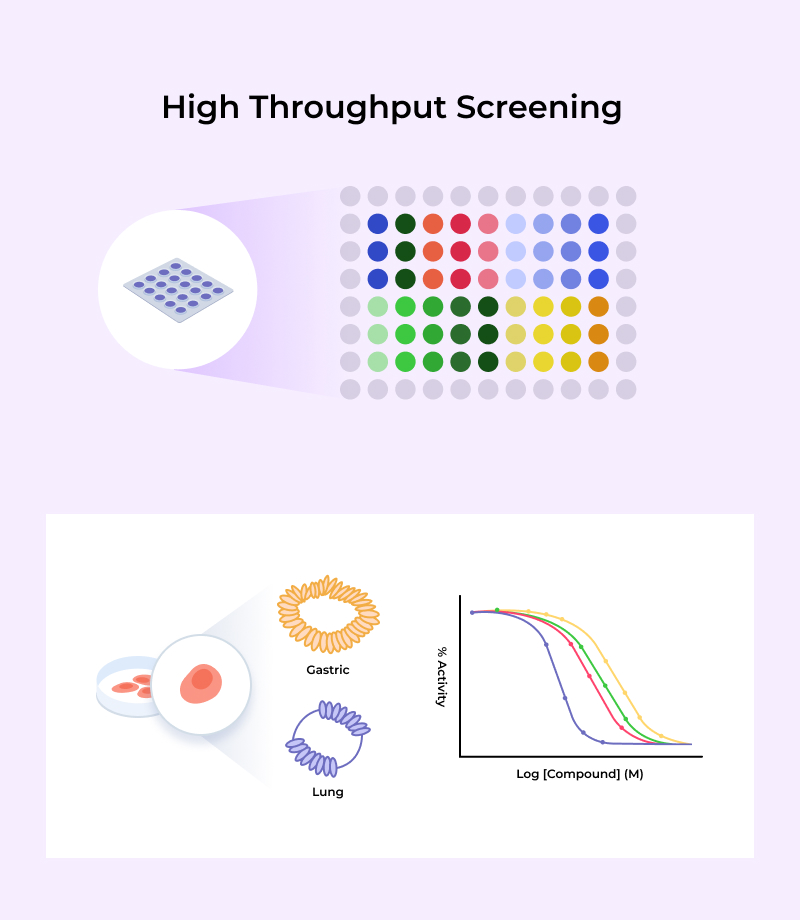
Evaluation of drug efficacy of tumor organoid-based immunotherapy
-
Step 04
Prescribing anticancer drugs to patients after screening ICI drugs