PODO Therapeutics was founded by combining Organoid Science’s organoid technology and Severance hospital’s clinical experience
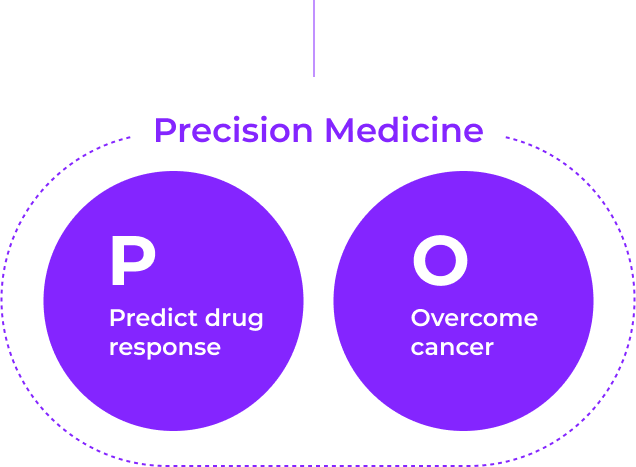
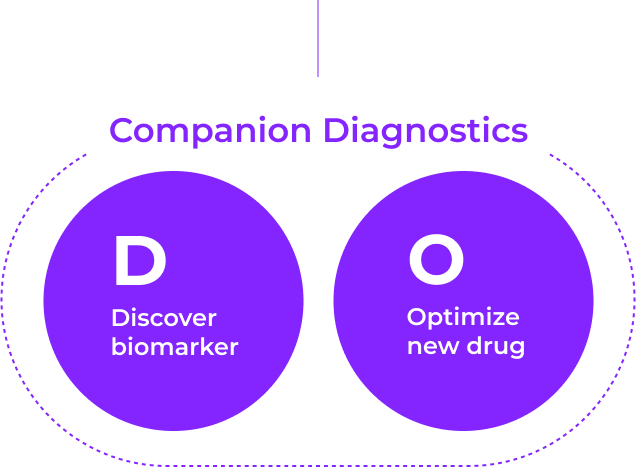
PODO Therapeutics has created an organoid platform applicable to precision medicine for anti-cancer treatment, biomarker discovery for new drug development, and companion diagnostics
PODO Therapeutics will open new horizons in anti-cancer treatment to extend the lives of patients suffering from cancer and help them recover their healthy lives
Vision
With cancer organoid
PODO Therapeutics
Organoid Enabled
Precision Oncology
History
2024
2023
-
06
- The selection of promising startups for the scale-up 100 in Jeonbuk State in 2024
- The establishment of Podotherapeutics' branch in Jeonbuk State
-
03
- 50 cases of anti-cancer drug susceptibility tests were conducted, including lung cancer, stomach cancer, colon cancer, and head and neck cancer, and clinical research was completed
-
01
- Acquired ISO 13485
-
11
- Conducted correlation analysis with clinical course of lung cancer and stomach cancer patients
-
10
- Obtained license for manufacturing In Vitro Diagnostic Medical Devices Regulation
- Obtained a manufacturing registration for In Vitro Diagnostic Medical Devices Regulation
-
08
- Pre-treatment reagent grade 1 medical device certification
-
07
- Establishment of organoid culture methods for each cancer type, including lung cancer, colon cancer, and stomach cancer
-
05
- Registered establishment of research institute
-
04
- Establishment of organoid standard analytical performance test method
-
03
- Establishment of co-culture technology protocol
-
01
- Completed IRB approval for 50 cases including lung cancer, colon cancer, stomach cancer, and head and neck cancer (Severance Hospital)